jún . 13, 2024 16:04 Back to list
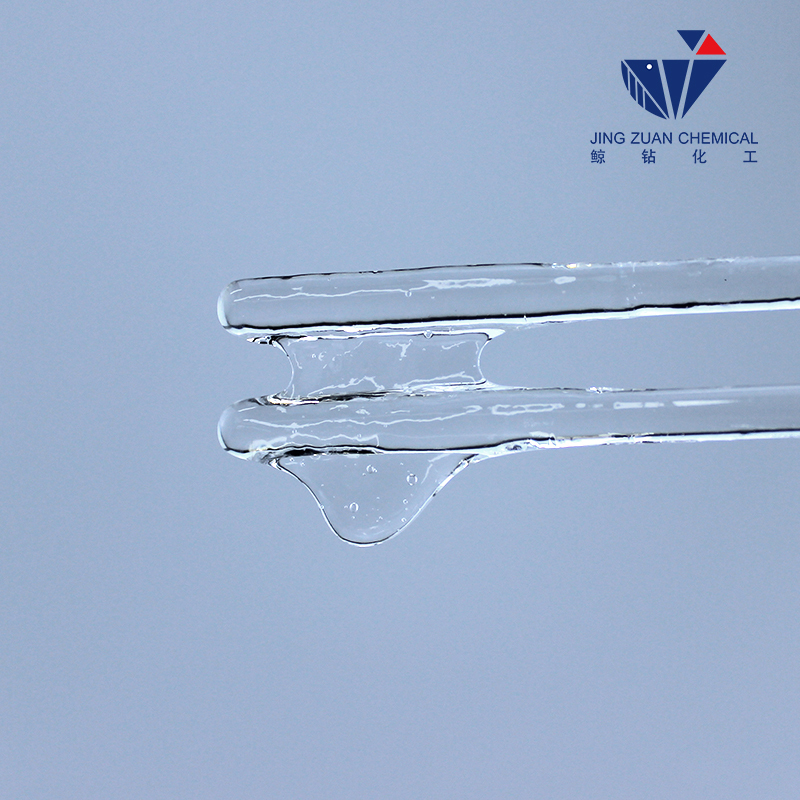
Hydroxyethyl cellulose's solubility in water is substantial.
The Solubility of Hydroxyethyl Cellulose in Water An In-Depth Analysis
Hydroxyethyl cellulose (HEC), a derivative of cellulose, is a widely used polymer in various industries due to its unique properties and versatility. A key aspect that governs its application is its solubility in water, which plays a crucial role in determining its efficacy as a thickener, stabilizer, or binder.
Hydroxyethyl cellulose's solubility in water is a result of the hydroxyethyl groups attached to the cellulose backbone. These hydroxyethyl groups make HEC more soluble in water compared to native cellulose, which is highly insoluble. The degree of substitution, or the number of hydroxyethyl groups per anhydroglucose unit, significantly influences the solubility. Higher degrees of substitution lead to better water solubility, as they enhance the hydrophilic nature of the polymer.
In pure water, HEC generally forms a clear, viscous solution at room temperature. The rate of dissolution depends on factors such as particle size, concentration, and temperature. Smaller particles dissolve more rapidly, while increasing temperature can enhance solubility by reducing the viscosity of water and increasing the kinetic energy of the molecules.
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution hydroxyethyl cellulose solubility in water. However, once fully dispersed, it forms a stable solution without any sedimentation or gel formation. In contrast, HEC dissolves more readily in hot water, making this method preferred for industrial applications where rapid mixing is essential.
It's worth noting that the presence of impurities, salts, or other substances can affect HEC's solubility. Some minerals and ions can interfere with the hydration process, potentially leading to precipitation or reduced solubility. Therefore, the quality of water used for dissolving HEC is critical for optimal performance.
In conclusion, the solubility of hydroxyethyl cellulose in water is a complex interplay between its chemical structure, environmental conditions, and processing parameters. Understanding these factors is vital for optimizing its use in diverse applications, ranging from construction materials to pharmaceutical formulations. Further research continues to explore ways to enhance its solubility and tailor its properties for specific end-use requirements.
hydroxyethyl cellulose solubility in water. However, once fully dispersed, it forms a stable solution without any sedimentation or gel formation. In contrast, HEC dissolves more readily in hot water, making this method preferred for industrial applications where rapid mixing is essential.
It's worth noting that the presence of impurities, salts, or other substances can affect HEC's solubility. Some minerals and ions can interfere with the hydration process, potentially leading to precipitation or reduced solubility. Therefore, the quality of water used for dissolving HEC is critical for optimal performance.
In conclusion, the solubility of hydroxyethyl cellulose in water is a complex interplay between its chemical structure, environmental conditions, and processing parameters. Understanding these factors is vital for optimizing its use in diverse applications, ranging from construction materials to pharmaceutical formulations. Further research continues to explore ways to enhance its solubility and tailor its properties for specific end-use requirements.
 The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution
The solubility of HEC in cold water is typically slow, often requiring agitation or heating to facilitate dissolution hydroxyethyl cellulose solubility in water. However, once fully dispersed, it forms a stable solution without any sedimentation or gel formation. In contrast, HEC dissolves more readily in hot water, making this method preferred for industrial applications where rapid mixing is essential.
It's worth noting that the presence of impurities, salts, or other substances can affect HEC's solubility. Some minerals and ions can interfere with the hydration process, potentially leading to precipitation or reduced solubility. Therefore, the quality of water used for dissolving HEC is critical for optimal performance.
In conclusion, the solubility of hydroxyethyl cellulose in water is a complex interplay between its chemical structure, environmental conditions, and processing parameters. Understanding these factors is vital for optimizing its use in diverse applications, ranging from construction materials to pharmaceutical formulations. Further research continues to explore ways to enhance its solubility and tailor its properties for specific end-use requirements.
hydroxyethyl cellulose solubility in water. However, once fully dispersed, it forms a stable solution without any sedimentation or gel formation. In contrast, HEC dissolves more readily in hot water, making this method preferred for industrial applications where rapid mixing is essential.
It's worth noting that the presence of impurities, salts, or other substances can affect HEC's solubility. Some minerals and ions can interfere with the hydration process, potentially leading to precipitation or reduced solubility. Therefore, the quality of water used for dissolving HEC is critical for optimal performance.
In conclusion, the solubility of hydroxyethyl cellulose in water is a complex interplay between its chemical structure, environmental conditions, and processing parameters. Understanding these factors is vital for optimizing its use in diverse applications, ranging from construction materials to pharmaceutical formulations. Further research continues to explore ways to enhance its solubility and tailor its properties for specific end-use requirements. Latest news
-
Versatile Hpmc Uses in Different Industries
NewsJun.19,2025
-
Redispersible Powder's Role in Enhancing Durability of Construction Products
NewsJun.19,2025
-
Hydroxyethyl Cellulose Applications Driving Green Industrial Processes
NewsJun.19,2025
-
Exploring Different Redispersible Polymer Powder
NewsJun.19,2025
-
Choosing the Right Mortar Bonding Agent
NewsJun.19,2025
-
Applications and Significance of China Hpmc in Modern Industries
NewsJun.19,2025
Related PRODUCTS